Laminaria: Uses and Risks
We inform, you decide.
What it is laminaria?
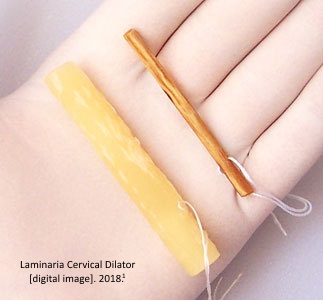
Laminaria is a kind of seaweed that can be sterilized, rolled and dried to create a thin stick or “tent” that can be inserted into the cervix to force dilation. There is a synthetic version of currently available in the United States called Dilapan-STM Also known as osmotic dilators, both are commonly used as a preparational step for second-trimester abortions. 1
How does laminaria work?
A laminaria tent is a small rod of dehydrated seaweed that when inserted in the cervix, rehydrates, absorbing the water from the surrounding tissue in the woman’s body. Laminaria expands up to ten times its original size, slowly opening the cervix, and when removed, creates easier access to the uterus. Steady pressure placed on the cervix by the tent also promotes the release of endogenous prostaglandins, causing contractions2 which readies the body for expulsion of the fetus.
When is laminaria used?
Laminaria is used mainly in second-trimester abortions (D&E). It is sometimes used in first-trimester abortions; however, compared to laminaria tents, other forms of cervical dilation are “associated with less discomfort and [are] preferred by women.”3 Depending on a woman’s health history, laminaria may not be the most effective method of cervical dilation, and each case must be considered independently. “Previous cesarean sections, cervical procedures and primigravidity [first pregnancy] were found to be risk factors for failure to achieve adequate cervical dilation after a single set of laminaria.”4
According to the University of California & Society for Family Planning, “No clear guideline currently exists for best practices involving cervical preparation for women planning dilation and evacuation at 20 weeks and greater.”5
What happens during the laminaria insertion procedure?
During the laminaria insertion procedure, the patient is awake and positioned for a pelvic exam. The practitioner will use a speculum to open the vagina. He or she will then clean the cervix and apply a numbing medication. Once this is done, the laminaria tent is inserted into the cervix. After the procedure, the woman should rest for a few minutes, and can dress and go home when she feels able. The entire procedure takes about 10 minutes. Cramping and spotting may occur following the insertion.6 Patients should return within 24 hours.
Women who have a laminaria tent placed must return for their follow up visit. Laminaria should not be left in place for over 24 hours due to increased risk of infection and other complications. Patients should never attempt to remove the tent on their own or pull the string.
Sometimes more than one laminaria tent is used to obtain the desired dilation, and “additional sets over 1–2 days may be needed in challenging cases.”7 No studies currently address whether using laminaria over the course of multiple days poses greater risk of infection.8
What are the risks associated with laminaria use?
According to WebMD, “The use of laminaria [is] likely unsafe when used to induce labor. It can cause serious side effects for both mother and child, including infection, rupture of the cervix, and infant death.”9 One study published in the Rocky Mountain Medical Journal showed that 1 in every 14 of women endured complications from laminaria use,10 and a study by the Swedish Association of Midwives stated: “Complications in the use of laminaria include difficulty of tent removal, cramps, and menstruation-like symptoms in 8-9% of patients.”11
Risks during the insertion procedure include:
- laminaria placed incorrectly
- laminaria needing to be removed due to pain
- allergic reaction to numbing medication
- spontaneous rupture of membranes (amniotic sac), increasing the risk for infection
- cervical rupture12
- convulsions
- uterus can move upward in the pelvis during insertion requiring stabilization with a tenaculum
Risks after the procedure include:
- “ballooning” or “dumbbelling” of the laminaria, where one end of the tent swells more significantly, making it difficult to remove13,14 (metal dilators may be needed to extract the laminaria; a tenaculum may also be used, in which case patients may experience “a transient sharp pain”)
- fragmentation and retention15,16 (pieces of the laminaria tent can break off when attempting to remove it, causing infection, pelvic pain, bleeding and even infertility17)
- migration to the uterine cavity
- failure of the uterus to contract
- hemorrhaging
- fever due to infection
- endometriosis
- severe cramping
- depression
- pelvic abscess
- cystitis (inflammation of the bladder)
Once the uterus is exposed to outside elements by the dilated cervix, the risk for infection and miscarriage increase significantly.18 According to the insert for Dilateria, a brand name of laminaria, if a patient changes her mind after the laminaria tent is in place, she should be advised that the “long-term effects on the pregnancy and the fetus are unknown. Fetal and maternal well-being in this situation cannot be assured.”19
If you’re considering a second-trimester abortion and want to know more about your abortion options, schedule a free consultation with us today. Let CompassCare’s professional, caring nurses help you make the right choice regarding your body and your future.

Medically Reviewed By:
NURSE TEAM LEADER, ROCHESTER
TAMARA M., BSN, RN
1 The Stevens Company Limited (2021). Laminaria Cervical Dilator 5mm Large. Retrieved June 2021 from Stevens.ca.
2 Dilapan-S® (2016). Introducing the Dilapan-S, fast-acting osmotic cervical dilator. Retrieved June 2021 from Medisafe Distribution, Inc. Youtube Channel.
3 Allen, R.H., Goldberg, A.B., (2017). Cervical dilation before first-trimester surgical abortion (<14 weeks’ gestation). Contraception; 76(2): 139-56
4 Ben-Ami, I., Stern, S., Vaknin, Z., Smorgick, N., Schneider, D., Halperin, R., (2015). Prevalence and risk factors of inadequate cervical dilation following laminaria insertion in second-trimester abortion-case control study. Contraception; 91(4): 308-312.
5 Uhm, S., University of California, Society of Family Planning (2018). Cervical Preparation with Mifepristone Prior to Osmotic Dilators. Retrieved June 2021 from U.S. National Library of Medicine, Clinical Trials.gov.
6 UCSF Medical Center (2021). Surgical Abortion: Second Trimester. Retrieved June 2021 from USCF Health.
7 Fox, M.C., Hayes, J.L., SFP, (2007). Cervical preparation for second-trimester surgical abortion prior to 20 weeks of gestation. Contraception; 76(6): 486-495.
8 Paul, M., Lichtenberg, S., Borgatta, L., Grimes, D., Stubblefield, P., Creinin, M.. (2009). Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. Wiley Pub. Ltd.
9 WebMD, LLC (2021). Laminaria. Retrieved June 2021 from WebMD.
10 Hern, W., (1975). Laminaria in abortion: Use in 1368 patients in first trimester. Roc Mtn Med J; 72(9): 390-395.
11 Jonasson, A., (1984). Laminaria–a modern cervix dilatation method with more than a 100-year history. Jordemodern; 97(6): 187-195.
12 Hamilton, S., Daw, E., (1991). Rupture of the Cervix During Gemeprost Middle Trimester Abortion. J Obstet Gynaecol;11(4): 295.
13 Gusdon, J.P., Jr, May, W.J., (1975). Complications caused by difficult removal of laminaria tents. Amer J Obstet Gynecol;121(2): 286-287.
14 Fox, M.C., Hayes, J.L., SFP, (2007). Cervical preparation for second-trimester surgical abortion prior to 20 weeks of gestation. Contraception; 76(6): 486-495.
15 Persad, M.D., Marinese, D., Osho, J., Muneyyirci-Delale, O., (2012). Challenging Removal of Retained Laminaria Fragment. J Gynecol Surg; 28(6): 443.
16 Nilsson, W., Mikheal, S., Kaplan, J., (2017). Chronic Pelvic Pain and Infertility Resulting from Unrecognized Retained Laminaria. Case Rep Obstet Gynecol; 2017(6345712): 1-3.
17 Allen, R.H., Goldberg, A.B., (2017). Cervical dilation before first-trimester surgical abortion (<14 weeks’ gestation). Contraception; 76(2): 139-56
18 Lee, S.M., Lee, K.A., Kim, S.M., Park, C.W., Yoon, B.H., (2011). The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta; 32(7): 516-521.
19 Cooper Surgical (2018). Dilateria (Laminaria Hyperborea): Instructions for Use. Retrieved June 2021 from 36999-B Model (1) (coopersurgical.com)